Retina Care Highlights January 2023

Keep up with the latest retinal care and clinical research highlights from Austin Retina Associates.
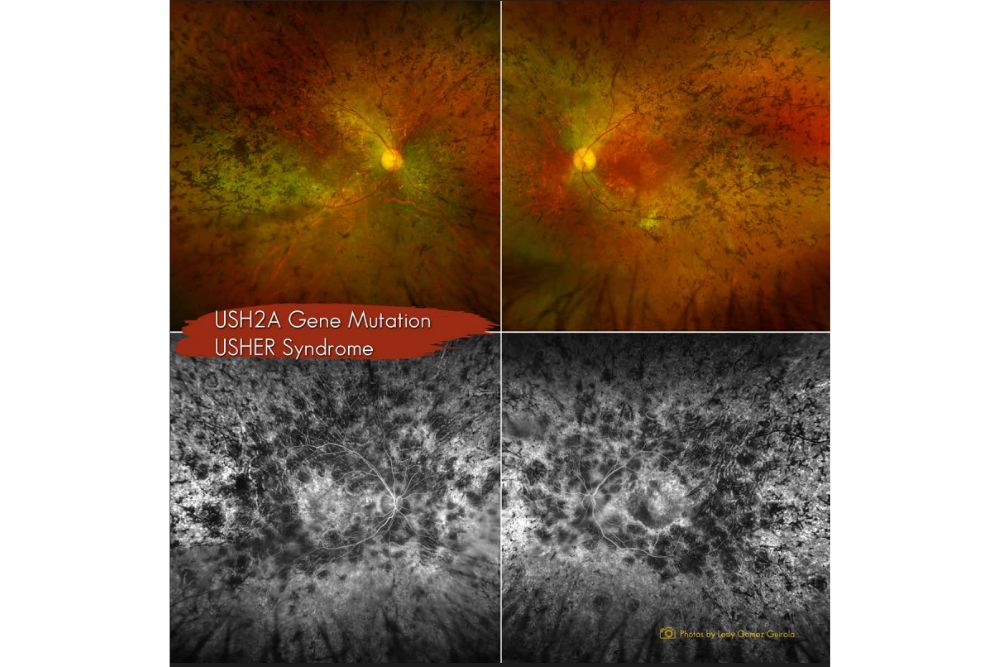
Case Report: Inherited Retinal Dystrophy
New Treatment for Wet AMD
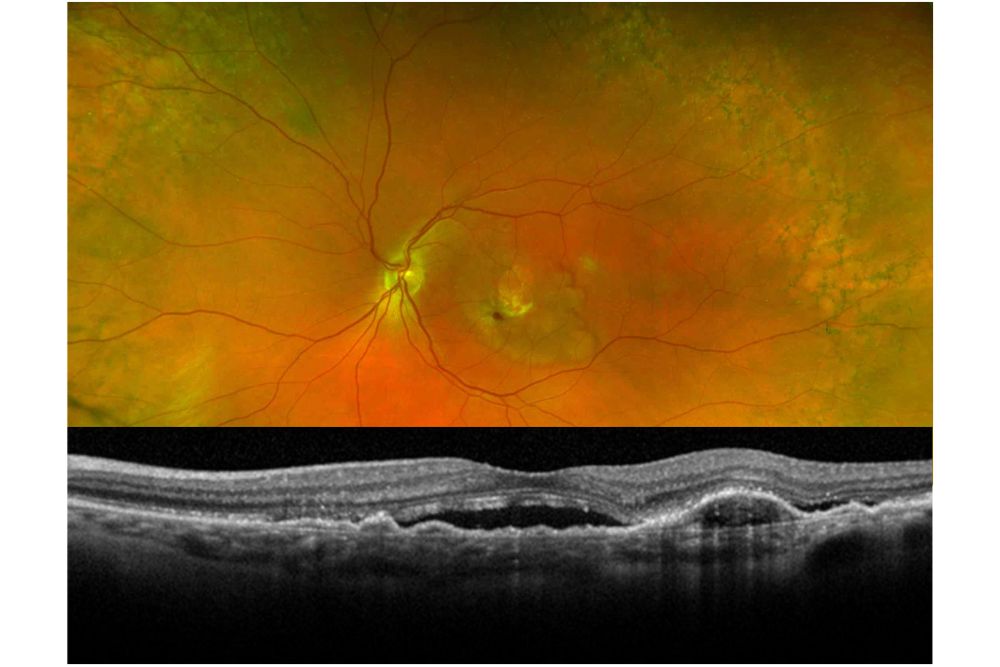
Neovascular (wet) age-related macular degeneration (AMD) showing hemorrhage (red spots in photo above), retinal pigment epithelial changes (white/brown spots in photo above), and subretinal fluid (black space in the cross-sectional optical coherence tomography (OCT) image below).
Age-related Macular Degeneration (AMD) is the leading cause of blindness in adults in the United States, affecting approximately 11 million people in the US. Occasionally, new blood vessels from a tissue layer underneath the retina called the choroid may leak or bleed in a process known as “wet” AMD. The new blood vessels are called “choroidal neovascularization” (CNV) and are the hallmark of wet AMD. Over time, CNV may result in scar tissue or cellular loss (atrophy), which are less reversible. While wet AMD only represents 10% of all AMD cases, it is responsible for the majority of severe vision loss. In order to preserve the best possible vision, wet AMD usually requires regular intravitreal injections of pharmacologic agents every 4-12 weeks.
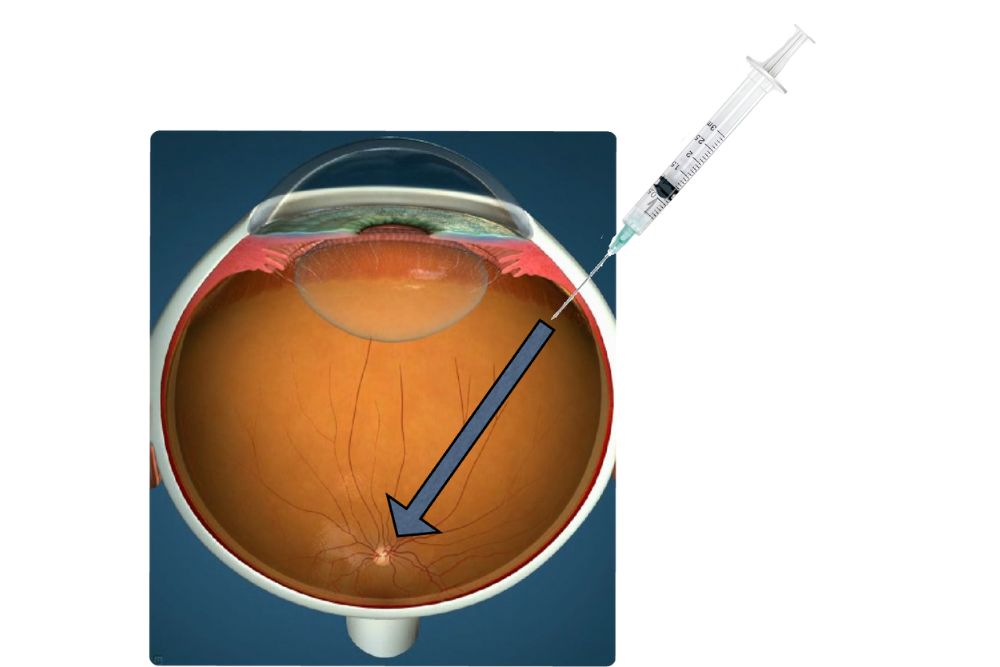
A schematic of an intravitreal injection, whereby medicine is inserted into the vitreous cavity to reach the retina for a therapeutic effect.
Austin Retina is participating in two clinical trials that could change this treatment approach for wet AMD. The trials explore a new technology called gene therapy where during a surgery the retina is given the instructions to make the same type of medicine patients receive in the clinic. This way, the body can make the drug in the retina right where it is needed on an ongoing basis with the goal of fewer injections in the clinic. Patients that enroll in the trial are randomized to receive either the new gene therapy approach or the current standard of care with intravitreal injections of ranibizumab (Lucentis) within the ATMOSPHERE trial at our Austin Main location or aflibercept (Eylea) within the ASCENT trial at our Round Rock location. If you’d be interested in learning more about these or other clinical trials at both our Main and Round Rock locations, please let your Austin Retina physician or staff member know.
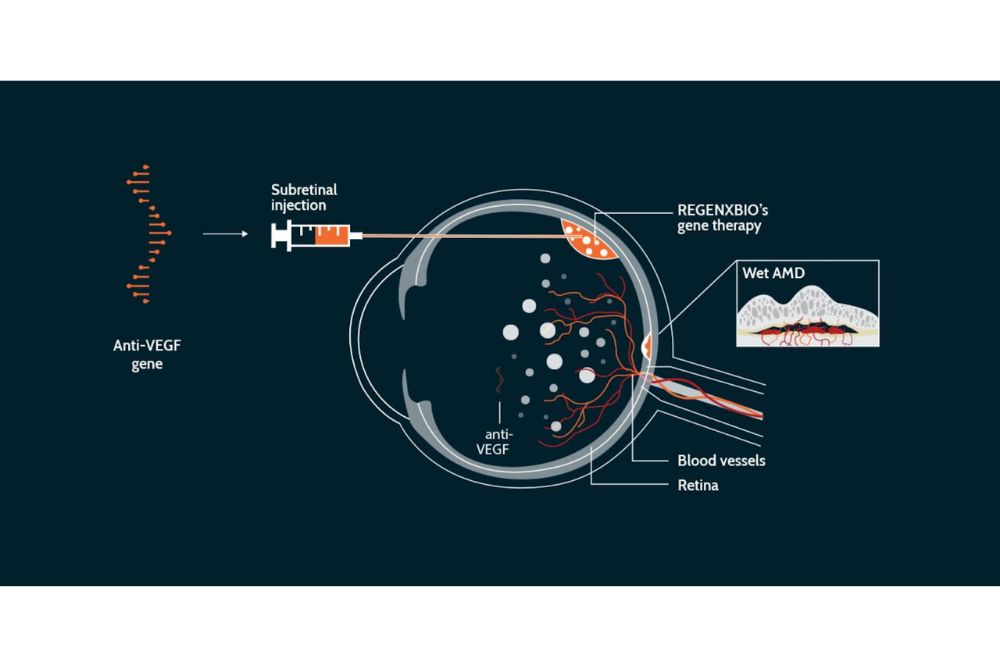
A schematic of the gene therapy treatment approach, whereby the “instructions” to make the medicine is given to the retina so that your eye can produce the necessary medicine on its own.