The Food and Drug Administration (FDA) approves new treatment for retinal disease

Wet age-related macular degeneration (AMD) and diabetic macular edema (DME) are leading causes of blindness in adults in the US. Both wet AMD and DME are commonly managed with injections of Vascular Endothelial Growth Factor (VEGF) inhibitors in the gel-like part of the eye (intravitreal) to decrease the growth and leakiness of blood vessels and improve vision.
On January 28, 2022, the FDA approved intravitreal faricimab (Vabysmo, Roche/Genentech) for the treatment of wet AMD1 and DME2. Vabysmo is the first drug of its kind to target two distinct biological pathways (Ang-2 and VEGF-A) that are strong contributors to retinal disease and vision loss. The FDA approved Vabysmo based on the results of four large (Phase 3) clinical trials1,2 that evaluated the durability of Vabysmo compared with aflibercept (Eylea, Regeneron) in untreated patients with wet AMD and mostly untreated patients with DME.
Whereas the majority of patients are currently treated with anti-VEGF injections every 1 to 3 months, the four clinical trials1,2 showed that the majority of patients treated with Vabysmo were able to maintain vision and reduce retinal fluid at 3 to 4 months between injections. For patients, this means the possibility of improved vision with less frequent treatment. The studies were not designed to determine which drug was superior, but rather to evaluate the efficacy, safety, and durability of Vabysmo using a treatment approach personalized to each patient. The rate of side effects (adverse events) were noted to be comparable between Vabysmo and Eylea.
Results from anti-VEGF injections in patient with wet AMD.
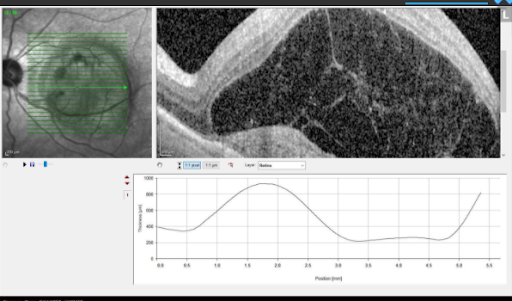
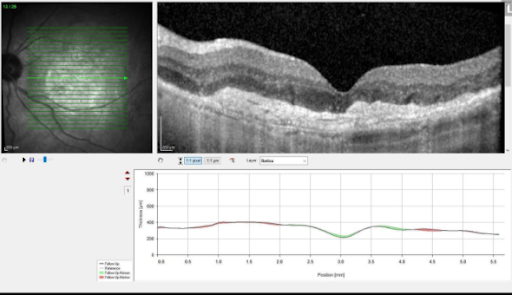
Austin Retina is committed to providing the most up-to-date and impactful treatments for retinal disease and plan to use Vabysmo in a manner personalized to your individual needs. We are committed to our patients achieving the best vision possible regardless of the therapy. If you have any questions or would like to learn more, please discuss with your doctor if Vabysmo might be right for you.
To schedule an appointment with one of our retinal specialists, please call 800-252-8259 or request an appointment online.
For the latest Austin Retina news, follow us on Facebook and Instagram.
References
-
Heier, Jeffrey S., Arshad M. Khanani, Carlos Quezada Ruiz, Karen Basu, Philip J. Ferrone,Christopher Brittain, Marta S. Figueroa, et al. 2022. “Efficacy, Durability, and Safety of Intravitreal Faricimab up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials.” The Lancet. https://www.sciencedirect.com/science/article/pii/S0140673622000101.
- Wykoff, C. C., F. Abreu, A. P. Adamis, and K. Basu. 2022. “Efficacy, Durability, and Safety of
Intravitreal Faricimab with Extended Dosing up to Every 16 Weeks in Patients with Diabetic Macular Oedema (YOSEMITE and RHINE) ….” The Lancet. https://www.sciencedirect.com/science/article/pii/S0140673622000186.

